Increasing complexity in the heart: How two related proteins control development of the heart
RBPMS and RBPMS2 regulate cell division and function during heart development
Researchers at the Max Planck Institute for Heart and Lung Research in Bad Nauheim have uncovered critical mechanisms that regulate embryonic heart development. The study discloses that two closely related RNA-binding proteins, RBPMS and RBPMS2, play vital roles in directing splicing of cardiac-specific RNAs. Disruption of this process results in severe structural malformations and halts heart development before birth. These findings have been recently published in the journal Developmental Cell.
During prenatal development, the heart undergoes critical molecular and structural changes, making it fit for a lifetime of heavy work. After birth, proliferation of cardiac cells stops and additional nuclei are acquired. At the same time, the contractile units, the sarcomeres, within cardiac muscle cells become stiffer. Disruptions or errors in this maturation process are frequently linked to congenital heart defects. To prevent congenital heart defects, a detailed understanding of the molecular mechanisms underlying cardiac cell maturation is essential.
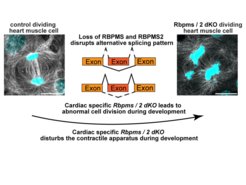
A key process for heart development is alternative splicing, which means that the primary RNA transcript copied from the genes is differentially processed, giving rise to different mature RNAs. RNA-binding proteins play a crucial role in this process by binding to specific sites on the RNA, thereby regulating its splicing. This regulation produces multiple protein isoforms, substantially increasing the diversity of the proteome.
Researchers from Thomas Braun's department at the Max Planck Institute for Heart and Lung Research have made significant advances in understanding heart maturation by studying two closely related RNA-binding proteins, RBPMS and RBPMS2, which act as splicing factors.
“First, we studied mice genetically engineered to lack only one of the two splicing factors of the RBPMS/RBPMS2 family. Remarkably, despite inactivation of RBPMS, mice showed normal heart development and normal heart function after birth”, says Shan Lin, the study’s first author. Next, Shan also inactivated RBPMS together with RBPMS2, which caused severe defects of heart development and function. “The simultaneous inactivation of RBPMS and RBPMS2 taught us that the loss of one gene can be compensated for by other genes during heart development”, explains Lin.
But which molecular processes are controlled by splicing proteins? "Our studies revealed that RBPMS and RBPMS2 play a key role in several fundamental processes of heart maturation," said Lin. The group discovered that RBPMS and RBPMS2 not only direct formation of isoforms for sarcomere proteins, but also correct formation of the spindle apparatus, which is essential for cell division. “When both proteins are inactive, cell division is disrupted”, explains Lin.
The research team now investigates the interplay of RBPMS and RBPMS2 with other splicing factors that are known causes for cardiomyopathy in human patients.
“In the long term, this could pave the way for novel therapeutic strategies,” says Thomas Braun, Director of the Department of Heart Development and Remodelling. “By clearly understanding the underlying causes, future RNA-based therapies will come into reach to restore or modulate RBPMS- and RBPMS2-regulated processes, offering targeted treatment for developmental defects resulting in congenital heart disease.”