The division of the indivisible: Heart regeneration possible after genetic reprogramming of cardiac muscle cells
Timed transient expression of stem cell factors enables cardiac regeneration in the mouse heart
The human heart has only limited self-healing powers and a limited regenerative capacity. A damaged heart muscle and the resulting loss of function can usually not be fully restored. The reason for this is that heart muscle cells in the adult organism have largely lost their ability to divide. Scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim, together with an international team of researchers, have now succeeded in enabling the regeneration of the damaged organ in mice by means of a temporally and spatially controlled reprogramming of heart muscle cells. The critical factor here is the controlled strength and duration of the reprogramming: if this is not optimal, regeneration fails to occur or tumors even form.
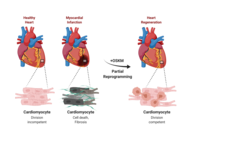
The mammalian heart is a high-performance organ. It reliably pumps blood through the body at a relatively high pressure, adapted to the respective demand. It is assumed that in the course of evolution this performance capacity was bought by the fact that the regenerative capacity of the organ was largely lost. The reason for this could be that the individual heart muscle cells are permanently and very closely connected to form a so-called electrical syncytium. The individual cells are interconnected via cell-cell contacts so that the electrical conduction necessary for cardiac contraction can take place in a controlled manner. New cardiac muscle cells produced by cell division could disrupt this physiological process and cause the heart to become out of sync. Accordingly, in contrast to organs with a high regenerative capacity, no divisible, regenerative stem cells are found in the heart. The disadvantage: If parts of the heart muscle are damaged, as is the case in a heart attack, for example, the heart lacks the self-healing powers. Functional regeneration of the damaged heart therefore does not usually take place.
Divisions of cardiac muscle cells are extremely rare. However, when this happens, it is associated with the activation of genes that are otherwise expressed only in early cardiac development. In other words, cardiac muscle cells rewind or dedifferentiate their developmental program under certain conditions. In this process, similar to prenatal development, a state is reached that should in principle allow cardiac muscle cells to divide. Unfortunately, the rewinding to an early state that is still capable of regeneration is only incomplete, similar to a tape that gets stuck when rewound. As a result, regeneration also gets stuck.
Scientists from the Department of "Development and Remodeling of the Heart" at the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now investigated in mice whether damaged heart muscle can be regenerated by means of forced temporary reprogramming of heart muscle cells. "Our approach was initially to use a cocktail of four stem cell factors to reprogram cardiac muscle cells in such a way that they first dedifferentiate, thereby triggering cell division," explains Johnny Kim, who led the study together with Thomas Braun. To do this, the group used transgenic mice in which the expression of the factors Oct4, Sox2, Klf4 and c-Myc, abbreviated as OSKM, can be specifically switched on and off in the heart. Artificial expression of OSKM enables, among other things, the reprogramming of somatic cells into induced pluripotent stem (iPS) cells from which all cell types of the adult body can arise. The development of induced pluripotent stem cells through OSKM expression earned the discoverer of the process, Shin'ya Yamanaka, the 2012 Nobel Prize in Physiology or Medicine.
"We harnessed the technology of reprogramming on cultured cardiac muscle cells that we had previously isolated from mice. We succeeded in specifically turning on OSKM expression in cardiac muscle cells. As a result, the cardiac muscle cells continued to dedifferentiate, gradually regaining their ability to divide," Kim said. The researchers then used the mice themselves to study the effect of turning on OSKM expression in the heart. "Indeed, after six days, we could see changes in the heart, including initial remodeling processes in the muscle and changes in the activity of a variety of genes, most notably genes involved in cell division and metabolic control. However, cardiac function was preserved when OSKM were turned off after one week." However, prolonged OSKM expression resulted in substantial irreversible changes in the hearts, up to and including impaired function. "If OSKM expression was permanently maintained, cardiac myocytes dedifferentiated to the extent that they completely lost their original identity and even cardiac tumors formed," said Yanpu Chen, first author of the study.
With these preliminary findings that it is in principle possible to control dedifferentiation and thus the division of cardiac muscle cells, the scientists investigated whether repair processes could also be triggered on the damaged heart. "In an infarct model, regeneration processes occurred with OSKM expression switched on in a controlled manner. Compared with control animals, the infarct scar was significantly smaller," Kim said. Repeated measurement of cardiac function over a longer period of time also showed that improvement in cardiac performance occurred when OSKM expression was briefly turned on before or shortly after a myocardial infarction.
According to Thomas Braun, director at the Max Planck Institute, the study demonstrates that therapeutic restoration of organ function in tissues with low regenerative capacity can be successful when cells that are incapable of division are given back their ability to divide. "However, the fact that we observed tumor growth in the heart under certain conditions also shows that there is still a long way to go before clinical application. Our findings point to a natural contrast between regeneration and cancer development. Well regenerating organs with a high cell division rate show a high cancer risk, poorly regenerating ones like the heart show a low cancer risk. If the regenerative capacity is increased therapeutically, the cancer risk also increases. Unfortunately, nothing in life is for free".
