Prof. Dr. Stefan Offermanns
Max-Planck-Institute for Heart and Lung Research
Dept. of Pharmacology
Research topics:
Metabolic and cardiovascular diseases
Tumor cell metastasis
G-protein-coupled receptors
We pursue both basic science projects and projects with the aim to solve medical problems. In basic sciences projects, we aim at understanding how cells of the vascular and metabolic system, the heart as well as tumor cells respond to chemical and mechanical stimuli to maintain homeostasis and to adapt to changing microenvironments. More medically oriented scientific projects deal with the mechanisms of pathophysiological processes and drug actions, in particular in the cardiovascular and metabolic system as well as in cancer with the option to carry the projects to a translational level.
Research areas:
1) Metabolic signaling in obesity and diabetes
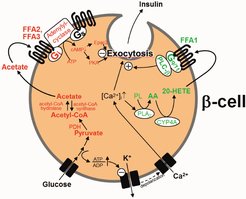
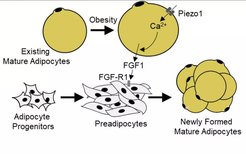
Metabolic disorders, such as obesity and type-2 diabetes, are risk factors for various diseases including cancer and cardiovascular disorders, and the number of obese and diabetic patients is dramatically increasing worldwide. In the past, we have identified positive and negative modulators of glucose-stimulated insulin secretion (Tang et al., 2015; Tunaru et al., 2018). We have also identified new regulatory mechanisms of adipocyte turnover and adipocyte function through autocrine regulatory loops (Ahmed et al., 2010), mechanosensitive processes (Wang et al., 2020a) as well as by anti-apoptotic signalling (Wang et al., 2020b). Future work will focus on the regulation of adipocyte and β-cell turnover and function in healthy and disease states as well as on understanding how metabolic disorders such as obesity promote cardiovascular disorders and cancer progression.
References
Wang L, Wang S, Shi Y, Li R, Günther S, Yuan Z, Liu E, Offermanns S. (2020b) YAP and TAZ protect against white adipocyte cell death during obesity. Nat. Commun. 11:5455
Wang S, Cao S, Arhatte M, Li D, Shi Y, Kurz S, Hu J, Wang L, Shao J, Atzberger A, Wang Z, Wang C, Zang W, Fleming I, Wettschureck N, Honoré E, Offermanns S. (2020a) Adipocyte Piezo1 mediates obesogenic adipogenesis through the FGF1/FGFR1 signaling pathway in mice. Nat. Commun. 11: 2303
Tunaru S, Bonnavion R, Brandenburger I, Thomas D, Scholich K, Offermanns S. (2018) 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1 (GPR40). Nat. Commun. 9: 177
Tang C, Ahmed K, Gille A, Lu S, Gröne HJ, Tunaru S, Offermanns S. (2015) Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 21: 173-177
Ahmed K, Tunaru S, Tang C, Müller M, Gille A, Sassmann A, Hanson J, and Offermanns S. (2010) An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11: 311-319
2) Tumor cell metastasis
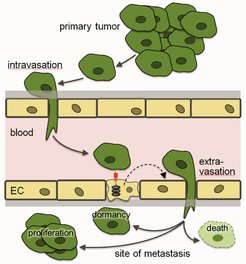
The metastatic cascade. EC, endothelial cell
Metastasis, the spreading of tumor cells mainly through the vascular system, accounts for the majority of cancer-associated deaths. However, the complex processes underlying tumor cell dissemination and growth of metastasis have remained poorly understood. Increasing evidence indicates that the microenvironment of the metastatic site plays a critical role in determining the progression of metastatic cancer. This includes the pre-metastatic niche which forms under the influence of ill-defined factors released from the primary tumor before tumor cells reach their metastatic sites. After tumor cells have arrived at places of metastasis, a local microenvironment forms and influences the fate of tumor cells which may die, grow or enter into a state of dormancy. Dormancy of metastasized tumor cells requires still poorly-understood conditions which support survival, suppress proliferation and may even provide resistance to therapeutic agents.
In the past, we have focused on the interaction of tumor cells with endothelial cells during the extravasation at metastatic sites (Schumacher et al., 2013; Strilic and Offermanns, 2017), and we identified mechanisms mediating tumor cell extravasation and efficient metastasis formation (Strilic et al., 2016). Currently, we plan to (1) further characterize mechanisms underlying tumor cell intravasation and extravasation, (2) characterize the pre-metastatic niche with a focus on the microvasculature and its role in promoting tumor cell metastasis and (3) identify local and systemic mechanisms which regulate tumor cell dormancy.
References
Nakayama A, Roquid KA, Iring A, Strilic B, Günther S, Chen M, Weinstein LS, Offermanns S. (2023) Suppression of CCL2 angiocrine function by adrenomedullin promotes tumor growth. J Exp Med. 220, e20211628
Strilic B, Offermanns S. (2017) Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 32: 282-293
Strilic B, Yang L, Albarrán-Juárez J, Wachsmuth L, Han K, Müller UC, Pasparakis M, Offermanns S. (2016) Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536: 215-218
Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. (2013) Platelet-derived nucleoides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24: 130-137
3) Mechanotransduction in the cardiovascular system and its relevance for vascular tone regulation and atherosclerosis
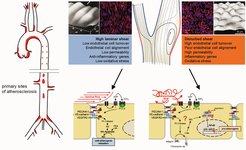
Atherosclerosis develops in areas of disturbed flow, which induces inflammatory signaling in endothelial cells. In contrast, laminar flow promotes atheroprotective signaling.
Fluid shear stress exerted by the flowing blood is crucial for the development of blood vessels but is also one of the major regulators of the vascular tone by inducing nitric oxide (NO) formation in endothelial cells, which relaxes vascular smooth muscle cells. Blood flow is also a key factor in the development of atherosclerosis, which mainly occurs in regions of arteries exposed to disturbances in fluid flow. Some of the endothelial mechanosensing and mechanotransduction mechanisms activated by laminar or disturbed flow to promote atheroprotective or atherogenic signaling have recently been identified. Understanding these fundamental processes provides new insights into the pathophysiology of cardiovascular diseases such as arterial hypertension and atherosclerosis.
Based on the analysis of basic signaling processes mediated by heterotrimeric G-proteins in vascular cells (Wirth et al., 2008; Korhonen et al., 2009, Althoff et al., 2012), we have recently identified some of the upstream mechanosensing and mechanosignaling mechanisms in endothelial cells, which involve the mechanosensitive cation channel Piezo1 and G-protein-coupled receptors (Wang et al., 2015; Wang et al., 2016; Albarrán-Juárez et al., 2018; Iring et al., 2019). Future work will aim at the identification of novel mechanosensitive and mechanotransducing processes in endothelial cells and other vascular cells which are differentially involved in anti-inflammatory (atheroprotective) and pro-inflammatory (atherogenic) signaling induced by laminar and disturbed flow, respectively. This includes the recently identified PKN2-mediated regulation of eNOS by laminar flow (Jin et al., 2021), the laminar flow-induced tenascin-X-mediated inhibition of endothelial-to-mesenchymal transition and atherosclerosis (Liang et al., 2022) as well as the role of PIezo1 in integrating low flow and leukocyte adhesion to promote leukocyte extravasation (Wang et al., 2022). The ultimate goal is to better understand how endothelial dysfunction contributes to vascular diseases in order to identify new approaches to prevent cardiovascular disorders at an earlier stage as it is currently possible.
References
Li R, Shao J, Jin YJ, Kawase H, Ong YT, Troidl K, Quan Q, Wang L, Bonnavion R, Wietelmann A, Helmbacher F, Potente M, Graumann J, Wettschureck N, Offermanns S. (2023) Endothelial FAT1 inhibits angiogenesis by controlling YAP/TAZ protein degradation via E3 ligase MIB2. Nat Commun. 14, 1980.
Wang S, Wang B, Shi Y, Möller T, Stegmeyer RI, Strilic B, Li T, Yuan Z, Wang C, Wettschureck N, Vestweber D, Offermanns S. (2022) Mechanosensation by endothelial PIEZO1 is required for leukocyte diapedesis. Blood 140: 171-183
Liang G, Wang S, Shao J, Jin Y, Xu L, Yan Y, Günther S, Wang L, Offermanns S. (2022) Tenascin-X Mediates Flow-Induced Suppression of EndMT and Atherosclerosis. Circ Res. 130: 1647-1659
Jin YJ, Chennupati R, Li R, Liang G, Wang S, Iring A, Graumann J, Wettschureck N, Offermanns S. (2021) Protein kinase N2 mediates flow-induced eNOS activation and vascular tone regulation. J. Clin. Invest. 131:e145734
Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Künne C, Sokol AM, Günther S, Martínez A, Fleming I, Wettschureck N, Graumann J, Weinstein LS, Offermanns S (2019) Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest. 130:2775-2791
Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, Offermanns S. (2018) Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 215: 2655-2672
Wang SP, Chennupati R, Iring A, Kaur H, Wettschureck N, Offermanns S. (2016) Endothelial Piezo1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Invest. 126: 4527-4536
Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S (2015). P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125: 3077-3086
Althoff TF, Albarrán Juárez J, Troidl K, Tang C, Wang S, Wirth A, Takefuji M, Wettschureck N, Offermanns S (2012) Procontractile G-protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling. J. Exp. Med. 209: 2277-90
Korhonen H, Fisslthaler B, Moers A, Wirth A, Habermehl D, Wieland T, Schütz G, Wettschureck N, Fleming I, Offermanns S. (2009) Anaphylactic shock depends on endothelial Gq/G11. J. Exp. Med. 206: 411-420
Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Őrsy P, Horváth B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind S, Offermanns S. (2008) G12/G13-LARG-mediated signalling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14: 64-68
4) G-protein-coupled receptors
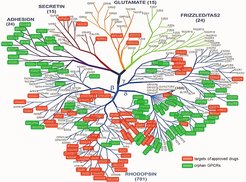
Human non-olfactory G-protein-coupled receptors (GPCRs). Established drug targets are in red, orphan GPCRs are in green.
G-protein-coupled receptors (GPCRs) are the largest receptor family in mammals, with about 800 receptors encoded in the human genome. Almost half of the receptors are olfactory receptors, whereas the remaining receptors respond to a wide spectrum of different ligands. For about 150 GPCRs no ligand has so far been described, making them “orphan GPCRs”. Every cell of the body expresses at least 15 to 20 GPCRs, which are involved in the regulation of all body functions. Their high ligand selectivity and their central and specific role in the modulation of physiological and pathophysiological processes make GPCRs ideal drug targets, and about a third of all approved drugs act through GPCRs.
In the past, we have identified new receptors activated by metabolites such as lactate, ketone bodies and free fatty acids (Tunaru et al., 2003; Ahmed et al., 2010; Offermanns, 2014; Blad et al., 2012). Using mouse genetics, we have been able to elucidate the role of several of these receptors in the pharmacological effects of drugs (Hanson et al., 2010; Lukasova et al., 2011), in the regulation of insulin secretion (Tang et al., 2015; Tunaru et al., 2018) as well as in endothelial flow sensing and blood pressure regulation (Wang et al., 2015; Iring et al., 2019). Using various approaches, we have identified new ligand receptor pairs (Tunaru et al., 2012; Le Mercier et al., 2021). Current work mainly focuses on orphan GPCRs and aims at the identification of new physiological GPCR ligands involved in the regulation of the cardiovascular and metabolic system as well as in cancer.
References
Le Mercier A, Bonnavion R, Yu W, Alnouri MW, Ramas S, Zhang Y, Jäger Y, Roquid KA, Jeong HW, Sivaraj KK, Cho H, Chen X, Strilic B, Sijmonsma T, Ralf Adams R, Schroeder T, Rieger MA, Offermanns S. (2021) GPR182 is an endothelium-specific atypical chemokine receptor that maintains hematopoietic stem cell homeostasis. Proc. Natl. Acad. Sci. USA. 118, e2021596118
Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Künne C, Sokol AM, Günther S, Martínez A, Fleming I, Wettschureck N, Graumann J, Weinstein LS, Offermanns S (2019) Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest. 130: 2775-2791
Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, Offermanns S. (2018) 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 9: 177
Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S (2015). P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125: 3077-3086
Offermanns S. (2014) Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) Receptors. Annu. Rev. Pharmacol. Toxicol. 54: 407-434
Blad CC, Tang C, Offermanns S (2012) G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug. Discov. 11: 601-619
Tunaru S, Althoff TF, Nusing RM, Diener M, Offermanns S (2012) Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc. Natl. Acad. Sci. U. S. A. 109: 9179-9184
Lukasova M, Malaval C, Gille A, Kero J, Offermanns S (2011a) Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 121: 1163-1173
Ahmed, K., Tunaru, S., Tang, C., Müller, M., Gille, A., Sassmann, A., Hanson, J., and Offermanns, S. (2010) An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11: 311-319
Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S. (2010) Keratinocytes express GPR109A and mediate epidermal effects of nicotinic acid and mono-Methyl fumarate via COX-2-dependent prostanoid formation J. Clin. Invest. 120: 2910-2919
Tunaru, S., Kero, J., Schaub, A., Wufka, C., Blaukat, A., Pfeffer, K., and Offermanns, S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352-355




